Abstract
Introduction: The interaction of menin with the epigenetic regulator KMT2A in acute leukemia with KMT2A rearrangement (KMT2Ar) or mutant NPM1 (mNPM1, NPM1c) leads to upregulation of leukemogenic genetic programs. Targeting the KMT2A-menin interaction downregulates aberrant expression of HOXA9 and MEIS1 and reverses leukemogenesis. KMT2Ar leukemia affects infants, children and adults and is associated with high rates of resistance to standard therapies. mNPM1 is found in 30% of pts with newly diagnosed AML. There are no approved targeted therapies for acute leukemia with KMT2Ar or mNPM1. SNDX-5613 (revumenib) is a potent, selective inhibitor of the menin-KMT2A interaction. In this first-in-human Ph 1 trial, we present the updated safety, pharmacokinetics, pharmacodynamics and efficacy of monotherapy with SNDX-5613 in pts with relapsed or refractory (R/R) acute leukemia, including those with KMT2Ar or mNPM1 (AUGMENT-101).
Methods: AUGMENT-101 includes 2 parallel dose escalations for pts not taking (Arm A) or taking (Arm B) strong CYP3A4 inhibitors. An early amendment allowed pts ≥30 days of age to enroll and restricted eligibility to only pts with KMT2Ar or mNPM1. SNDX-5613 was administered orally, Q12h, in 28-day cycles. We employed a Rolling 6 design with expansion at efficacious doses. Pts who received ≥1 dose of SNDX-5613 were included in safety analyses; only pts with KMT2Ar or mNPM1 were included in efficacy analyses.
Results: Between 11/2019 and 03/2022, 68 pts with R/R acute leukemia (including 60 pts with KMT2Ar or mNPM1 assessed locally) enrolled on Arm A or B of the study (data cutoff 31Mar2022). Median age was 43 years (range, 1-79) across age groups, and 51 years among adults (range 19-79), with 82% AML (n=56), 16% ALL (n=11) and 2% MPAL (1 pt). Among pts enrolled, 46/68 (68%) had KMT2Ar, 14/68 (21%) had mNPM1, and 8/68 (12%) had other genotypes. Pts were heavily pretreated with a median of 4 (range, 1-12) prior lines of therapy and 46% had prior transplant. DLTs in both arms of the dose-escalation phase were asymptomatic grade 3 QTc prolongation. No ventricular arrhythmias were reported. 226 and 276 mg Q12h for Arm A, and 113 and 163 mg Q12h for Arm B, met the pre-specified criteria for the recommended ph 2 dose. PK analyses showed dose proportional exposure was achieved in both arms with steady state achieved in 48 hours and no evidence of drug accumulation.
Consistent with the established mechanism of action, menin inhibition by SNDX-5613 resulted in downregulation of the critical leukemogenic target genes MEIS1, HOXA9 and PBX3 and an increase in expression of genes associated with differentiation such as ITGAM (CD11b) and CD14. FLT3, a putative transcriptional target of MEIS1, and a gene frequently co-mutated in KMT2Ar or mNPM1 leukemia, was downregulated post-treatment. Differentiation syndrome, all grade 2, was reported in 11 pts (16%) and resolved with steroids and/or hydroxyurea. Grade ≥3 treatment-related adverse events (TRAEs) other than DLT included diarrhea (3%), fatigue (3%), anemia (3%), tumor lysis syndrome (2%), neutropenia (2%), thrombocytopenia (2%), hypercalcemia (2%) and hypokalemia (2%). No TRAEs led to treatment discontinuation or death.
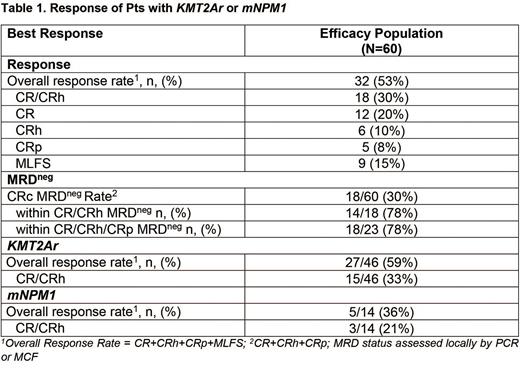
Responses were seen in both arms and at multiple dose levels. The overall response rate (CR+CRh+CRp+CRi/MLFS [ORR]) in the efficacy population was 53% (32/60); the rate of CR+CRh+CRp was 38% (23/60) with a CR/CRh rate of 30% (18/60 [95% CI: 18.8, 43.2]). The MRD negative rate among pts with CR/CRh/CRp was 78% (18/23) (Table 1). Pts with KMT2Ar or mNPM1 had an ORR of 59% (27/46 pts) and 36% (5/14 pts), respectively. Of responding pts, 3/5 with mNPM1 and 2/27 with KMT2Ar had FLT3 co-mutation. Median follow up in the efficacy population was 4.6 months (range 0.3 - 21.9); median duration of response was 9.1 months (95% CI: 2.7-NR) and median overall survival was 7 months (95% CI: 4.3-11.6; range 0.3-21.9+). 12 pts proceeded to allogeneic stem cell transplant. Pts with wild-type KMT2A and NPM1 had genotypes not predicted to be susceptible to menin inhibition and did not respond to therapy with SNDX-5613.
Conclusion: These data establish menin inhibition with SNDX-5613 as a successful therapeutic strategy for acute leukemia with KMT2Ar or mNPM1. Treatment was well-tolerated, associated with a low frequency of ≥grade 3 TRAEs and durable remissions in pts with leukemia refractory to multiple prior lines of therapy. Enrollment on Ph 2 of AUGMENT-101 is ongoing.
Disclosures
Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Aldoss:Kite: Consultancy; Autolus Limited: Consultancy; AbbVie: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Amgen: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau. DiPersio:Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Stone:Jazz: Consultancy; Gemoab: Consultancy; Epizyme: Consultancy; Foghorn Therapeutics: Consultancy; Janssen: Consultancy; Astellas: Consultancy; BerGenBio: Consultancy; Apteva: Consultancy; Kura Oncology: Consultancy; Novartis: Consultancy; OncoNova: Consultancy; Aprea: Consultancy; Boston Pharmaceuticals: Consultancy; Arog: Consultancy, Research Funding; BMS: Consultancy; Actinium: Consultancy; Syndax: Consultancy; GSK: Consultancy; Innate: Consultancy; Elevate Bio: Consultancy; Abbvie: Consultancy, Research Funding; Syntrix: Consultancy; Syros: Consultancy; Takeda: Consultancy. Arellano:Kite, a Gilead Company: Consultancy, Research Funding; Syndax Pharmaceuticals: Consultancy. Thirman:AbbVie, AstraZeneca, Celgene,Janssen, Pharmacyclics, Roche/Genentech: Consultancy; AbbVie,Gilead Sciences,Janssen,Merck,Pharmacyclics, Syndax, TG Therapeutics, Tolero Pharmaceuticals.: Consultancy, Research Funding. Patel:Pharmacyclics/Janssen: Consultancy; Pfizer/EMD Serono: Consultancy; Acerta Pharma: Research Funding; ADC Therapeutics: Research Funding; Agenus: Research Funding; Aileron Therapeutics: Research Funding; AstraZeneca: Research Funding; BioNTech AG: Research Funding; Boehringer Ingelheim: Research Funding; Celgene: Research Funding, Speakers Bureau; Checkpoint Therapeutics: Research Funding; CicloMed: Research Funding; Clovis Oncology: Research Funding; Cyteir Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; EMD Serono: Research Funding; Evelo Therapeutics: Research Funding; FORMA Therapeutics: Research Funding; Genentech/Roche: Honoraria, Research Funding, Speakers Bureau; Gilead Sciences: Research Funding; GlaxoSmithKline: Research Funding; H3 Biomedicine: Research Funding; Hengrui Therapeutics: Research Funding; Hutchison MediPharma: Research Funding; Ignyta: Research Funding; Incyte: Research Funding; Jacobio: Research Funding; Janssen: Honoraria, Research Funding; Klus Pharma: Research Funding; Kymab: Research Funding; Loxo: Research Funding; LSK Biopartners: Research Funding; Lycera: Research Funding; Macrogenics: Research Funding; Merck: Research Funding; Millennium: Research Funding; Mirati Therapeutics: Research Funding; Moderna Therapeutics: Research Funding; Pfizer: Honoraria, Research Funding; Placon: Research Funding; Portola Pharmaceuticals: Research Funding; Prelude Therapeutics: Research Funding; Ribon Therapeutics: Research Funding; Seven and Eight Biopharmaceuticals: Research Funding; Syndax: Research Funding; Taiho Pharmaceutical: Research Funding, Speakers Bureau; Takeda: Research Funding; Tesaro: Research Funding; TopAlliance BioSciences Inc: Research Funding; Vigeo: Research Funding; ORIC: Research Funding; Artios: Research Funding; Treadwell: Research Funding; MabSpace: Research Funding; IgM Biosciences: Research Funding; Puretech: Research Funding; BioTheryX: Research Funding; Black Diamond Therapeutics: Research Funding; NGM Biopharmaceuticals: Research Funding; Novartis: Research Funding; Nurix: Research Funding; Relay Therapeutics: Research Funding; Samumed: Research Funding; Silicon Therapeutics: Research Funding; TeneoBio: Research Funding; Zymeworks: Research Funding; Olema: Research Funding; Adagene: Research Funding; Astellas: Research Funding; Accutar Biotech: Research Funding; Compugen: Research Funding; Immunogen: Research Funding; Blueprint Pharmaceuticals: Research Funding; Pharmacyclics: Honoraria; Bayer: Honoraria; Adaptive Biotechnologies: Honoraria; Exelixis: Speakers Bureau; ION Pharma: Other: Leadership. Dickens:Tempus: Consultancy. Shenoy:NHLBI: Other: DSMB member; California Institute of Regenerative Medicine: Consultancy; Artio Med Inc: Consultancy; Takeda: Honoraria; Graphite Bio: Consultancy; Bristol Myers Squibb: Consultancy; Aruvant Sciences Inc.: Other: Chair, DSMB. Rosen:Syndax: Current Employment. Bagley:Syndax: Current Employment. Meyers:Syndax: Current Employment. Madigan:Syndax: Current Employment. Ordentlich:Syndax: Current Employment. Gu:Syndax: Current Employment. Smith:Syndax: Consultancy. McGeehan:Syndax: Current Employment. Stein:Syndax: Consultancy, Research Funding; Bayer: Research Funding; Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen, AbbVie, Seattle Genetics, and Biotheryx: Consultancy; PTC Therapeutics and Syros: Membership on an entity's Board of Directors or advisory committees; PinotBio, Bristol Myers Squibb, Jazz Pharmaceuticals, Foghorn Therapeutics, Blueprint Medicines, Gilead Sciences, Janssen Pharmaceuticals: Consultancy; Auron Therapeutics: Current equity holder in private company; Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.